More Information
Submitted: January 30, 2025 | Approved: February 08, 2025 | Published: February 10, 2025
How to cite this article: Luisetto M, Kaled E, Mashori GR, Ferraiuolo A, Fiazza C, Cabianca L, et al. GELS as Pharmaceutical Form in Hospital Galenic Practice: Chemico-physical and Pharmaceutical Aspects. Arch Surg Clin Res. 2025; 9(1): 001-007. Available from:
https://dx.doi.org/10.29328/journal.ascr.1001084.
DOI: 10.29328/journal.ascr.1001084
Copyright license: © Luisetto M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Gels; Chemical-physical properties; Pharmaceuticals; Galenic laboratory; Excipients; Antidote emergency medicine; Toxicity; Pediatry; Drug shortage
GELS as Pharmaceutical Form in Hospital Galenic Practice: Chemico-physical and Pharmaceutical Aspects
Luisetto M1*, Edbey Kaled2, Mashori GR3, Ferraiuolo A4, Fiazza C5, Cabianca L6 and Latyschev OY7
1IMA Academy, Independent Researcher Applied Pharmacologist, Hospital Pharmacist Manager, Galenic Lab, PC Area 29121, Italy
2Professor of Physical Chemistry, Libyan Authority for Scientific Research, Libya
3Professor, Department of Medical & Health Sciences for Women, Peoples University of Medical and Health Sciences for Women, Pakistan
4Hospital Pharmacist, Pc Area, Italy
5Medical Pharmacologist, Hospital Pharmacist Manager, Independent Researcher PC Area, Italy
6Medical Laboratory Turin, City of Health, Italy
7President, IMA Academy International, India
*Address for Correspondence: Luisetto M, IMA Academy, Independent Researcher Applied Pharmacologist, Hospital Pharmacist Manager, Galenic Lab, PC area 29121, Italy,Email: [email protected]
This work aims to describe the chemical-physical properties of various GELS used as galenic forms in hospital pharmacy practice. After an overview of the excipients and method used three preparations are reported. LAT GEL is used as an anesthetic in an emergency (pediatry ) in treating little Traumatic lacerations of the skin and scalp, calcium gel is used as an antidote for fluoride acid burns, and Lidocaine viscose 2% oral gel is used in some pathological conditions like severe esophagitis in onco - hematological patients after radiotherapy or chemotherapy. The galenic role in the situation of some drug shortages was also analyzed.
This kind of galenic preparation is semisolid and for cutaneous use: a dispersed system with a dispersed phase (liquid) and a gelified dispersant phase (solid). The liquid is trapped by the gelificant structure (a tridimensional structure) giving a characteristic viscosity. The liquidi is transformed in gel using gelificants and can be hydrophilic or hydrophobic. This macromolecule must have two properties: great solvation and the capacity to join together in their contact points.
In the hydrophilic gel, there is the swelling of a polymer in water, with a tridimensional structure that incorporates the water. They contain water, glycerin, propylene glycol, and gelificants. For example, starch glicerolate gel (starch /glycerin/water at 10:70:20 w/w) is used as a cutaneous emollient for dry cute. The hydrophobic gels (lipogels) are based on oleic phase Gelified with polymers, silica gel, hydrogenated ricin oil, and beeswax (in example based on low molecular weight glicole dispersed in liquid paraffin).
In pharmacy, this product is used to achieve an optimal percutaneous drug delivery for prolonged absorption or a topic Release (xylocaine viscose oral gel). Among the advantages of this pharmaceutical form, are: easy formulation, not dirty, not greasy, good shelf life (with preservants), easily spreadable, generally accepted by patients, and low cost. They can be aqueous or alcoholic. The gelificant can have a concentration from about 0,5% - 2.5% since 5% for methylcellulose. This preparation needs a perfect solubilization of the API and of the other components [1-9].
Classification of gels
- Inorganic (allumine idroxide, bentonite).
- Organic (carbossipolimetilene) - carbomer,poloxamer, PVP.
- Idrogel (silica, pectin, metilcellulose, alginate,Carbossimetilcellulose CMC, adragant gum, carraghenan).
- Organogel (unguenta PEG, plastibase).
Common characteristics required of these excipients are the inert property, atoxic, and compatibility with the APIs to be mixed. Xerogel is obtained by eliminating the water from a hydrophilic gel, then from this, it is possible -to obtain the gel by adding the water.
Reology: it is the study of the scroll property of fluids and solids.
Viscosity: resistance of fluid to the scroll. Depends on the tridimensional reticular structure of the gel
The gels have non-Newtonian properties.
Gelification point: it is the concentration of gelificant. Under this concentration is not possible to have the gelification.
Excipients used in the preparation of industrial gels
- Antimicrobial preservatives, Antioxidant, Chelating, Humectants, Fragrances, emulsifiers
- gelling agents, Permeation enhancers, Co solvents, Polymers, Colour, Adhesives, Adsorbents
- Air displacement agents, Anticaking agents, Antifoaming agents
- Antifungal preservatives, Binders, Buffering agents, Flocculating agents, Lubricating agents
The hydrophilic polymers are namely, Guar gum, pectin, alginates, carrageenin, xantan gum, gelatin, amido, carpool, nitroso, and HPMC.
The preservatives are: parabens 0,2%, benzoic acid 0,2%, chlorocresol 0,1%
Preparation methods
It depends on the kind of gelificant agent to be used:
- Carbopol: slow add in water under great mixing, because it is an acrylic acid derivate for gelification is needed to change PH (since PH is 7) by adding a small amount of NaOH (the great number of -COOH become -COO- with an increase in electrostatic repulsion).
- When the bases are in a small amount the PH variation can be observed with the PH litmus test or by observing the gelification.
An example is alcoholic disinfectant acrylic gel for hands.
Carbopol 940 0,5 gr, triethanolamine (1:1 in water) as needed, alcohol 96 grades 75 ml, glycerin 5 ml, H2O2 3 ml And water q.b .100 ml.
- Add alcohol water and glycerin, then H2O2 mixing and the carpool.
- Add a small amount of the bases and check the PH or the gel produced.
- Gelatin: dispersing it in hot water and then cooling it
- Methylcellulose: disperse in hot water (80-90 grades) under mixing, add the rest of the water, and cool.
- It needs to avoid grumes adding the gelificant at “rain”, and wet before the powder with alcohol or propylene glycol then add the hot water.
- Preservatives: the water gel must to preserved by adding parabens 0,1% to avoid the growth of microorganisms.
- The presence of alcohol helps but the gels can dry more rapidly.
- Hydroxyethyl cellulose gel: boil the water to reduce possible microbic charge.
- In becker Add water and glycerin, Disperse HEC and the preservant.
- The glycerin is used to increase the solvation and wettability of HEC.
GEL base for cutaneous FU XII ED use: Caramellosa sodium 5 gr, glycerol 85% 10 gr, depurated water at final 100 gr.
- It can be a substitute caramellosa with Idroxietil cellulose 2,5 gr working at hot temperatures.
- Add in a beaker at veil the the IEC in hot water, then add the glycerol mixing slowly with the wand to avoid air incorporation, and let cool for gelification.
- It needs a preservative.
Note: The APIS must be solved before in the right solvent in which they are soluble and then gelifing the system (for the water-soluble APIs using water, alcohol, glycerol) and oil for liposoluble.
Piroxicam hydroalcholic gel: piroxicam 500 mg, idroxipropil cellulose 1,75 gr, propilenglicol 4,1 gr
- Polysorbate 80 1,7 gr, isopropyl alcohol 70% since total weight of 100 gr.
- Add idroxipropilcellulose to isopropyl alcohol mixing since gelification, a part mix the piroxicam with propylene glycol and polysorbate 80 and then using the geometric mixing method add the gel previously prepared.
Other examples of formulation
Miconazole 20 mg/g oral gel (for Candida oral infection)
- Idrossimetilpropil cellulosa; glicerol; depurated water qb 100 g.
Gel idrofobic (oleogel):
- The base is usually liquid paraffin with polyethylene or oil and fats verified with colloidal silica aluminum or zinc soaps. The gelification is obtained with additives like hydrogenated ricin oil, stearate, and micronized silica at 3% - 8%.
Silica colloidal Lipogel:
- Silica colloidal (micronized) and g 5.
- Sweet almond oil g 95.
- Then add oil mixing slowly without intake air.
- Rest for 1 hour to get the gel.
- It can be added fragrance 0,5%
- 0,5% - 1% di tocoferol acetate or 0,01% di BHT as antioxidant.
Control of the galenic gels:
- Verify the following: pH, labels, amount required.
Sterility and microbiological quality
The gels can be considered and labeled nonsterile or sterile according to the procedure followed (aseptic technique, use of filter 0,22 micron, and the kind of galenic lab).
Industrial products require specific tests for microbic contamination and allergizant effects. In the Pediatric emergency department it is of interest the formulation of LAT GEL:
According to E. Benelli, et al. 2013, “Local anesthesia LA for lacerations is obtained by perilesional injections of lidocaine, which are painful. Ready-made anesthetic gels that can be directly put on the laceration with no pain and that have anesthetic and hemostatic power are available. Between these, LAT gel (lidocaine 4%, adrenaline 0.05%, tetracaine 0.5%) was found to be safe and effective.”
Lat gel (lidocain, adrenalin, tetracain) composition, Store at 2 0C - 8 0C, expiration date: 90 days if used preservants. Pediatric use, Not considered sterile ( See Galenic mortuary SIFO Italy: required powder hood for the preparation, needed normal lab glassware, not required millipore filter, 60 days expiry time).
Lidocaine is classified as Poison in the Italian Pharmacopeia XII ed., so it must be stored in a closed cabinet with a key (the responsibility of the pharmacist).
Topical lidocaine adrenaline tetracaine (LAT gel) versus injectable buffered lidocaine for local anesthesia in laceration repair Ernst, et al. [14] summarized the following: “The objective of the study work was to compare topical lidocaine adrenaline tetracaine (LAT gel) with injectable buffered lidocaine with epinephrine regarding pain of application or injection and anesthesia effectiveness. 66 patients were entered, 33 in the LAT gel group and 33 in the injectable buffered lidocaine group. Injection was found to be significantly more painful than the application of gel (p < 0.001) [14].
For anesthesia effectiveness, there was no difference according to patients (p = 0.48) or physicians (p = 0.83) for topical vs injectable forms. The numbness of sutures causing pain was not statistically different in the 2 groups (p = 0.28). LAT gel was compared favorably with injectable buffered lidocaine for local anesthesia LA effectiveness and was significantly less painful to apply. It may be the preferred local anesthetic for this reason.”
Calcium gel:
- APIs: calcium gluconate 2,5% e lidocaine 2%, propilen glicole, nipagin, gelificant hydroxyethylcellulose, water.
- Expiry time: 30 days (but 90 days with preservatives).
- Not available in commerce in Italy, the antidote for cutaneous intoxication by fluridric acid classified as priority II, needed availability in two hours in emergency departments.
- C-Gel combines with and neutralizes the fluoride ion present in HF acid.
- It must be used as a source of calcium in the gluconate end, not the calcium chloride because it is an irritant for the acute lesion area.
- After the preparation, it can be used aluminum tubes to be filled and closed.
- In an emergency if not available it can be prepared by mixing lidocaine gel 1-2, 5% plus calcium gluconate in adequate parts following the indication of a Poison center.
Calcium gel composition: Calcium gluconate 2,5 gr; Lidocaine cloridrtate 2 gr; Hydroxyethyllcellulose 5 gr; Propilen glicole 15,5 gr; Nipagin 0,10 gr; Water PI 75 GR.
Physiological Factors Affecting Skin Penetration: Skin integrity, level of hydration, temperature, Regional variation, Traumatic or pathologic injury, and Cutaneous drug metabolism.
The formulation factors affecting the specific skin penetration are namely, penetration enhancer, occlusivity, API concentration, pH, solubility, and surfactant used.
Other examples of gels are- ophthalmic gels, antimicrobial gels, gynecological gels, cosmetic gels, phytotherapy gels, toothpaste, anti-sunburn gels, and others.
In the pharmaceutical industry, gels can beaApis or phytotherapy, metronidazole, ketoprofen, diclofenac, heparin, antihistaminics, tretinoin, azelaic acid, TST, Aloe, Arnica, Calendula, and others.
With an observational method, some relevant literature related to the topic of this work is reported and analyzed. Various figures (Figures 1-15) help in the general understanding. Some classic formulations of gel are included in the study. An experimental project is provided and then after all this, a global conclusion is submitted.
Database source
All literature comes from Pubmed or other relevant database.
Figure 1: The liquid gel.
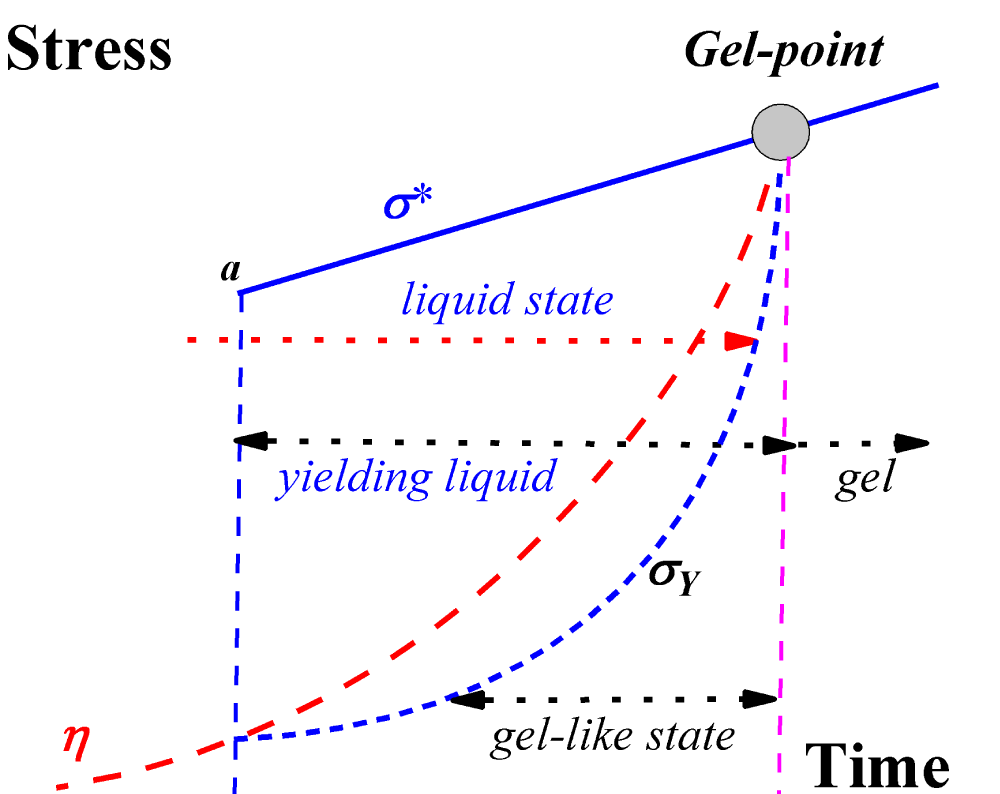
Figure 2: A rheological model of gelation. Here, the viscosity = η, the stress = σ* [10].
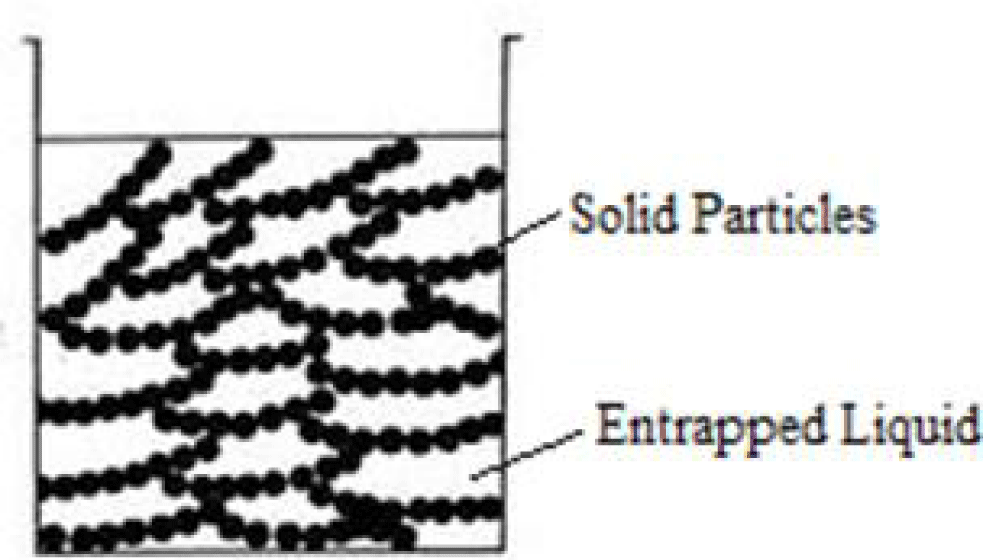
Figure 3: Solid particles and entrapped liquid during gel formation [11].
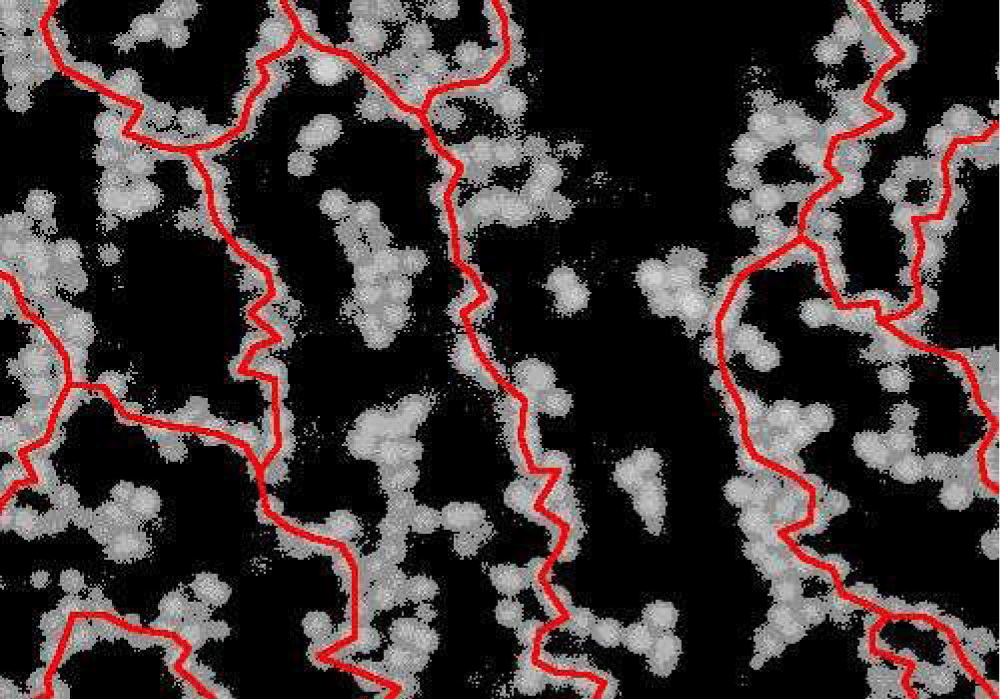
Figure 4: An image of a colloidal gel taken with a confocal microscope. Directed chains of particles (shown in red) that span the whole system are required for gels like this to form [12].
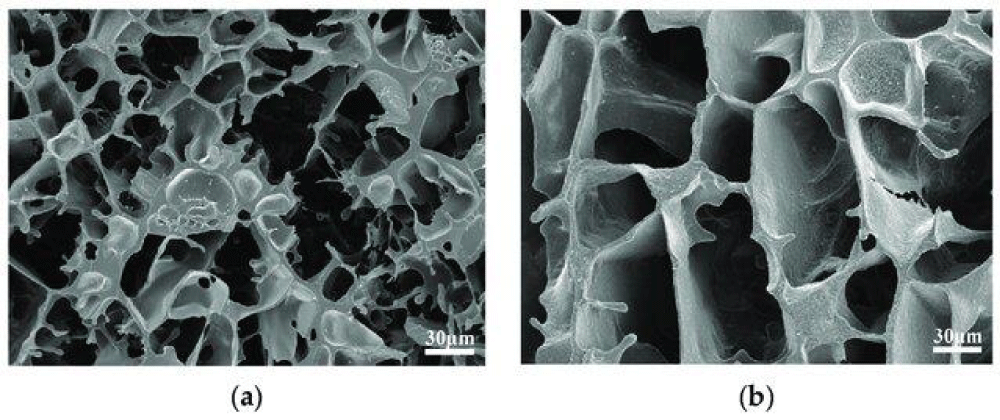
Figure 5: Microscopic morphological characterization of gel. (a) Gel structure after aging at 130 0C; (b) Gel structure after aging at 130 [13].
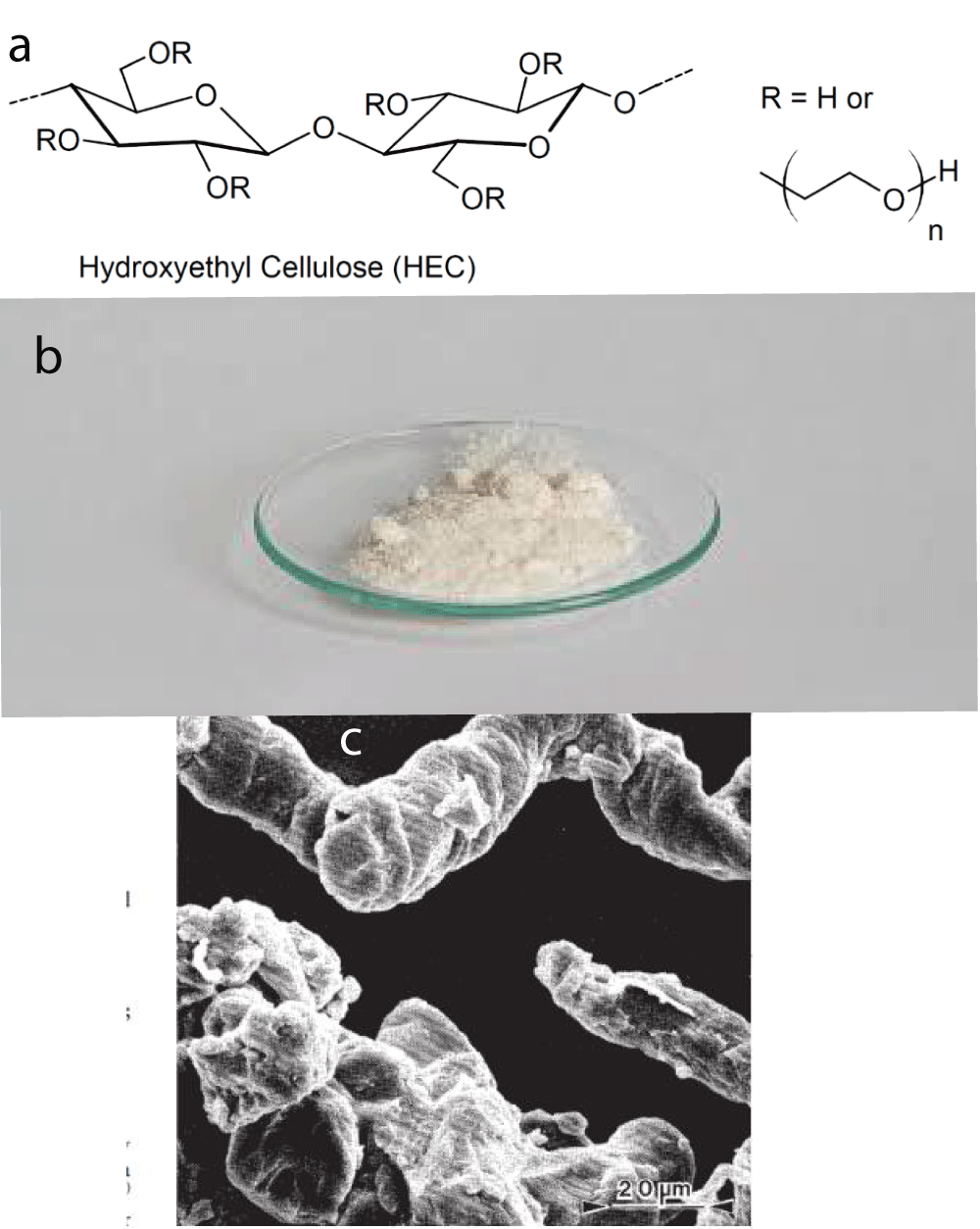
Figure 6: a: The structure of HEC-Hydroxyethylcellulose. b: Natrosol hydroxyethylcellulose. c: Hydroxyethylcellulose (magnification 600x) From Handbook of pharmaceutical excipients 6th edition [9].
Figure 7: The alcoholic disinfectant acrylic gel for hands.
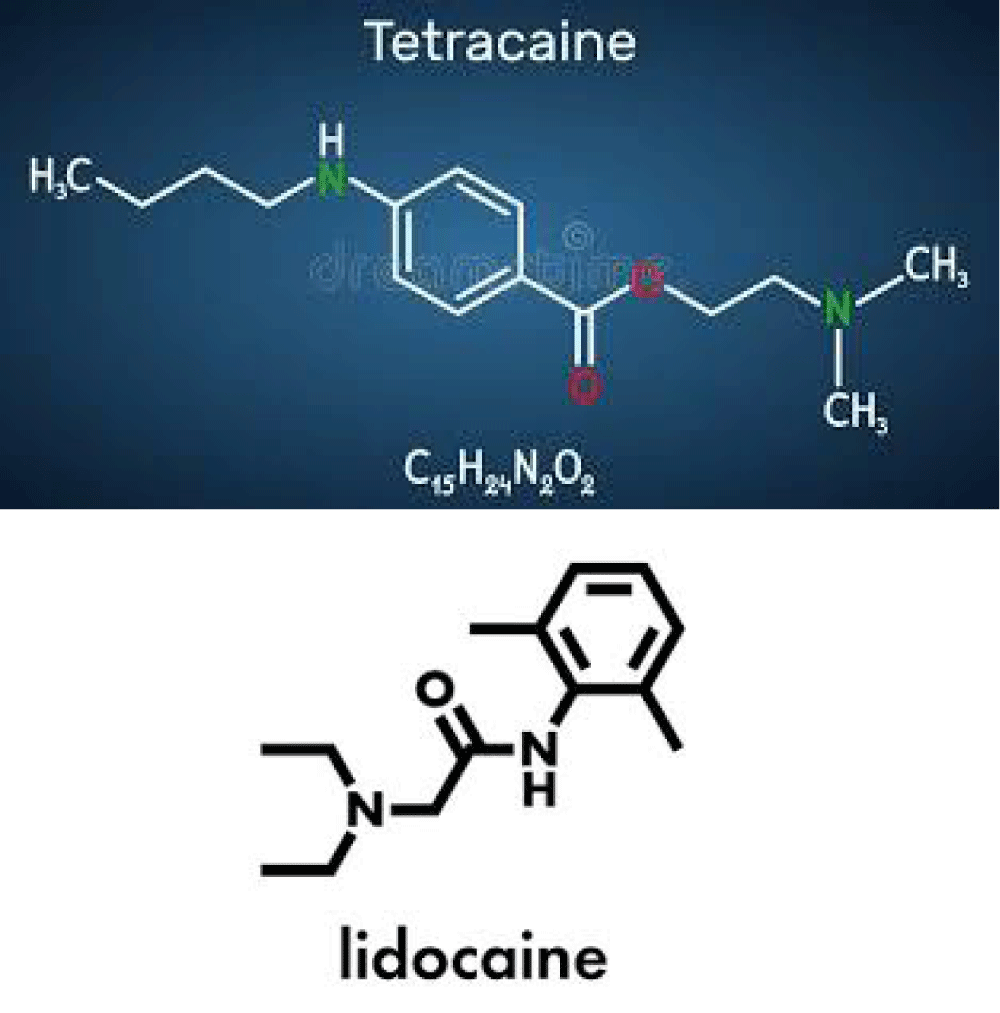
Figure 8: Tetracain and lidocain formula.
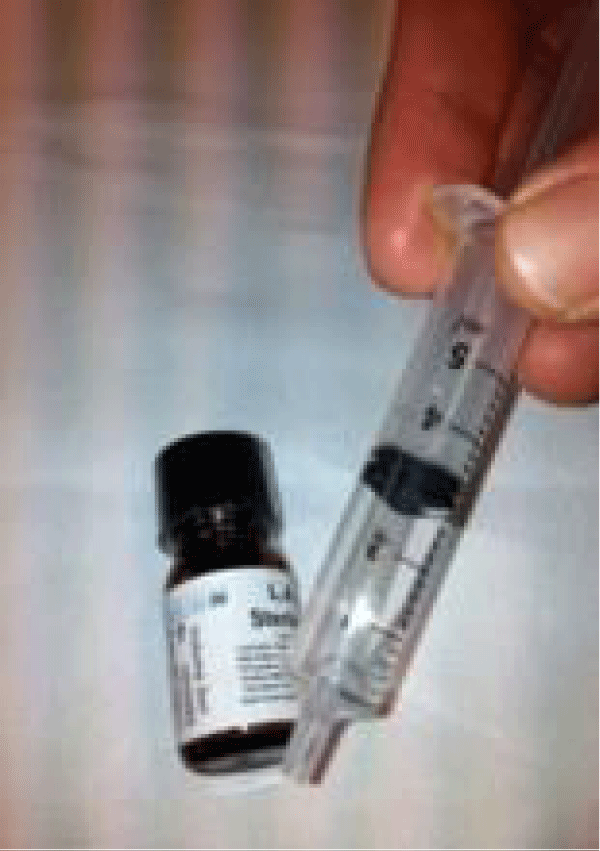
Figure 9: LAT gel from “LAT gel topical anesthetic for pediatric wounds” [8].
Figure 10: An image from Nicoletti, et al. 2013 (PMID: 24734325) [7].
Figure 11: The aluminum tubes for galenic use.
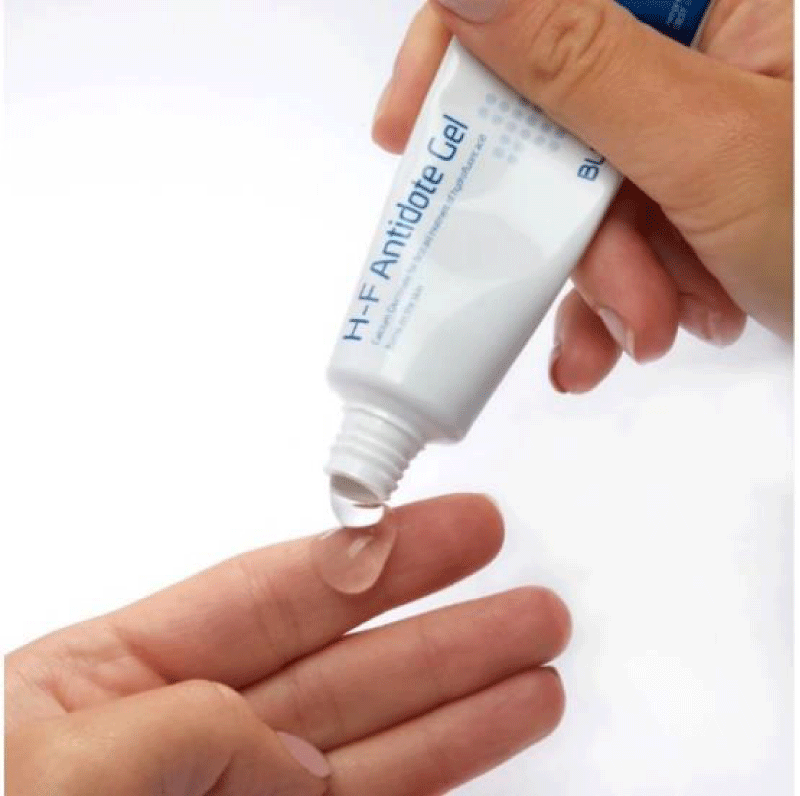
Figure 12: The H-F Antidote gel, a Calcium gel (storage 2 0C - 8 0C).
Figure 13: The Xylocaine viscose, a 2% oral gel.
Figure 14: The clindamycin phosphate gel, an antibiotic gel.
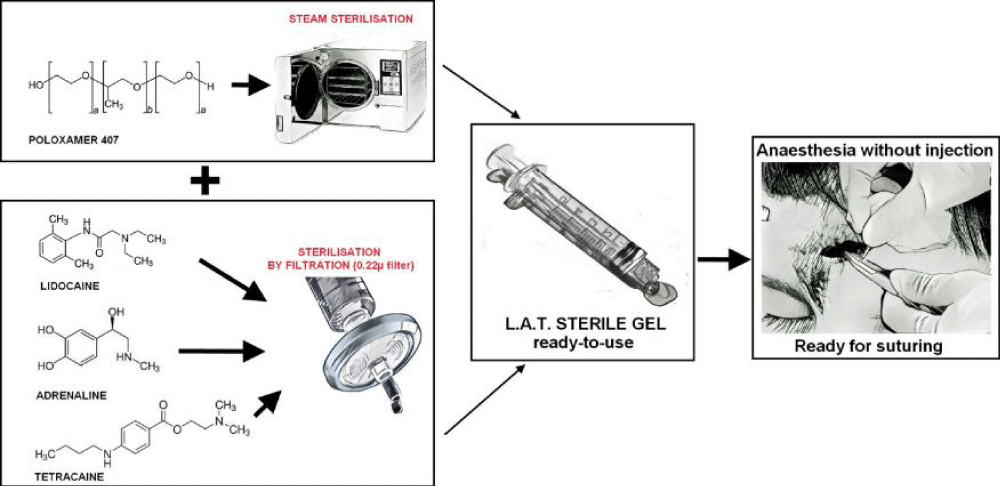
Figure 15: L.A.T. gel made with Poloxamer 407 and a two-step sterilization method [2].
Vandamme, et al. [15] stated that LAT gel is a topical anesthetic that can be applied on lacerations before suturing. It is considered easy to use and less painful than infiltrative anesthesia. Its use in laceration management has been studied the most in younger children. We aimed to describe the potential value of the use of LAT gel in older children and adults with simple lacerations.
LAT gel is a valuable alternative to infiltrative anesthesia for laceration repair. Its use should not be limited to children. The application of LAT gel seems to be specifically suitable for the short lacerations (<4 cm), lacerations located on the head, and simple finger lacerations [15].
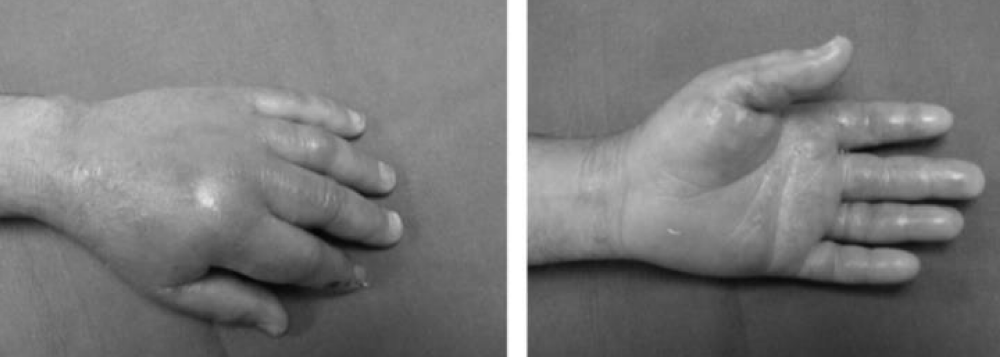
Chick and Borah [16], reported Hydrofluoric acid (HF) is used extensively as an industrial cleaning agent for metals and glass. Many workers are injured by cutaneous contact of the acid with exposed skin surfaces, particularly hands. Hydrofluoric acid HF burns are characterized by delayed onset of symptomatology with skin ulceration, and severe pain may be of extended duration. Treatment of hydrofluoric acid HF burns traditionally consists of local infiltration or intraarterial injections of calcium solutions. These injections are painful and frequently require retreatment. A new treatment utilizing a topical gel of calcium carbonate is described. 9 patients have been treated for hydrofluoric acid burns of the hand with calcium carbonate gel applied topically and covered with occlusive glove dressings. A gel slurry is compounded from calcium carbonate tablets and K-Y Jelly. The calcium carbonate gel technique was successfully utilized in nine patients with no further need for injection therapy. In these patients, pain relief was obtained within 4 hours of treatment, with no further progression of skin ulceration. No reconstructive procedures were required in any patient, and only one patient did not return to full-duty work within 1 week. There were no long-term sequelae from burns treated with this topical therapy, except for 1 patient, who presented 24 hours after the burn and developed a digital tip neuroma that was excised [16].
Yamashita, et al. [17] explored an Oral viscous lidocaine OVL is useful for the treatment of symptoms induced by oral inflamed mucosa, such as radiation- or chemotherapy-induced mucositis. The toxic reactions associated with an accidental overdose have been reported in pediatric cases. We report a case of lidocaine toxicity in a 22-year-old man during frequent viscous lidocaine use for severe painful tongue ulcers. The toxic symptoms developed when the amount of oral viscous lidocaine OVL exceeded 240 ml per day. The serum lidocaine concentration associated with this use was 6.7 microg/ml. The toxic symptoms continued despite the serum lidocaine SL concentration below the toxic level after the start of a diluted preparation, which contained a half-dose lidocaine should consider the risk of lidocaine toxicity in cases of frequent viscous lidocaine use, and determine the serum concentrations of lidocaine and its metabolites [17].
The Food and Drug Administration has issued a Public Health Advisory to create an alert for consumers, patients, healthcare professionals, and caregivers. FDA is issuing this advisory to alert you to the potential hazards of using skin-numbing products: topical anesthetics, for cosmetic procedures. These Topical Anesthetics (TA) contain anesthetic drugs such as lidocaine, tetracaine, benzocaine, and prilocaine in a cream, or gel [18].
- Use a topical anesthetic approved by the FDA.
- Use a topical anesthetic TA that contains the lowest amount of anesthetic drugs possible that will relieve your pain. Ask your doctor if the amount of anesthetic drugs in the cream is needed or advised for your procedure. Ask your doctor what side effects are possible from these drugs and how to lower your chance of having life-threatening side effects from these drugs.
- Be sure you receive instructions from your doctor on how to safely use the topical anesthetic TA. Apply as little of the cream to cover the affected skin area for the briefest period possible. If wrapping or covering the skin with any type of material or dressing is recommended or desired, be aware that this step can increase the chance of side effects [18].
Elad, et al. [19] proposed the following: The additional use of topical anesthetics/analgesics can help make this task easier in times when oral mucositis OM is present such as viscous xylocaine, dyclonine or diphenhydramine for those with allergies to esters and amides; topical analgesics TA such as doxepin and opioids may also reduce pain thus facilitating oral care (19).
Product
- Kind of preparation: LAT geL a calcium gel(formulation with preservative- NIPAGIN, not labeled as sterile)
- Time of observation: 1 year
- Place: Public hospital Galenic lab Pc AREA
- Normative rules followed: D.M. del 18 novembre 2003
- Outcomes measure: ADR and FV reports
- Results: no reported major non-conformity related quality and safety of the preparation during the time of observation.
Procedure
- Verify the concentration of the gelificant to be used to obtain the final product with the characteristic. Work at a hot temperature (80 °C - 90 °C) if needed.
- Mix slowly or Not to incorporate air
- Add preservants, especially for aqueous-based gels.
- Use water PI or bolied dep. Water to avoid microbiological problems.
- Work in an aseptic way, using the filter for the final product of 0.22 microns.
- Expiry time: For calcium gel- 30 days if without preservant, 90 days if the formulation has Nipagin.
- Check the final container closing systems and the right labeling (poison label, and sterile only if required or followed specific procedure) and the expiration date.
- To be specified also on the label the need to store at 2-8 °C.
- Calcium gel is provided in an aluminum tube for cream gel and then closed, instead, the LAT GEL is provided in a syringe do 5 ml with a luer lock closing.
- The xylocain viscose 2% gel is provided in flac. 200 ml dark glasses with closing system.
In hospital practice, various gels are requested from the galenic laboratory of the pharmacy. This galenic form makes it possible to deliver the APIS needed in the right way. Because of its specific chemical -physical properties some considerations must be taken into consideration. Dissolve APIS in their solvent first when possible before adding the gelificant.
Due to their specific chemical-physical and pharmaceutical properties, the gel such as galenic form is a good source to veiculate APIs for the needed use. Especially in a water-based gel, it is necessary to use preservatives to avoid microbial growth. The lipogel is useful as an antioxidants. The APIs must be dissolved r in water or alcohol or glycerin- if water-soluble or in oil- if liposoluble. The gelificant must be employed at the right concentration, without incorporating air during the mixing phase and working at a hot temperature. The glycerin is used to increase the solvation and wettability. The excipient must be added slowly. While using lidocaine oral gel for pediatrics, adverse effects must be considered.
- Benelli E, Zanon D, Bressan S, Facchina G, Vecchiato K, Pusceddu S, et al. Anesthetic efficacy of LAT gel for superficial wounds in a pediatric population. Already done?!. Doctor and Child. 2013;16(5). Available from: https://www.medicoebambino.com/?id=RIC1305_10.html
- Zanon D, Volpato C, Addobbati R, Loiacono S, Maestro A. Stability of a novel Lidocaine, Adrenaline, and Tetracaine sterile thermosensitive gel: A ready-to-use formulation. Eur J Pharm Sci. 2019;136:104962. Available from: https://doi.org/10.1016/j.ejps.2019.104962
- SIFO Galenic Code. 2010. Available from: https://www.sifoweb.it/biblioteca-sifo/altre-edizioni/4275-codice-di-galenica-sifo.html
- DM 18 November 2003 Italy; Procedure for preparation of magistral and officinal. DM Salute 18.11.2003 (Simplified NBP (NORME DI BUONA PREPARAZIONE) rules).
- NBP official pharmacopeia. Italy. Available from: https://www.iss.it/web/iss-en/official-pharmacopoeia-of-the-italian-republic
- Sheskey PJ, Hancock BC, Moss GP, Goldfarb DJ, editors. Handbook of Pharmaceutical Excipients. 9th ed. London: Pharmaceutical Press; 2022. ISBN 9780857113757.
- Nicoletti G, Pellegatta T, Scevola S. Current affairs on the electrical safety of equipment used in surgery and interventional medicine. G Ital Med Lav Ergon. 2013;35(3):176-82. Italian. Available from: https://pubmed.ncbi.nlm.nih.gov/24734325/
- Donald C, White A. LAT gel topical anesthetic for pediatric wounds. 2016. Available from: https://rightdecisions.scot.nhs.uk/nhs-tayside-ed-guidance/paediatrics/paediatric-analgesia/lat-gel/lat-gel-topical-anaesthetic-for-paediatric-wounds/
- Rowe RC, Sheskey PJ, Quinn ME, editors. Handbook of Pharmaceutical Excipients. 6th ed. London; Chicago; Washington, DC: Pharmaceutical Press; American Pharmacists Association; 2009. Available from: https://adiyugatama.wordpress.com/wp-content/uploads/2012/03/handbook-of-pharmaceutical-excipients-6th-ed.pdf
- Malkin AY, Derkach SR, Kulichikhin VG. Rheology of Gels and Yielding Liquids. Gels. 2023;9(9):715. Available from: https://doi.org/10.3390/gels9090715
- Gels. Available from: https://thefactfactor.com/facts/pure_science/chemistry/physical-chemistry/gels/11922/
- Capellmann R, Schmiedeberg M. Insights into gels. Available from: https://phys.org/news/2016-07-insights-gels.html
- Li X, Fu M, Hu J. Preparation and Performance Evaluation of Temperature-Resistant and Salt-Resistant Gels. Gels. 2024;10(5):337. Available from: https://doi.org/10.3390/gels10050337
- Ernst AA, Marvez-Valls E, Nick TG, Mills T, Minvielle L, Houry D. Topical lidocaine adrenaline tetracaine (LAT gel) versus injectable buffered lidocaine for local anesthesia in laceration repair. West J Med. 1997;167(2):79-81. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC1304430/
- Vandamme E, Lemoyne S, van der Gucht A, de Cock P, van de Voorde P. LAT gel for laceration repair in the emergency department: not only for children? Eur J Emerg Med. 2017;24(1):55-59. Available from: https://doi.org/10.1097/mej.0000000000000298
- Chick LR, Borah G. Calcium carbonate gel therapy for hydrofluoric acid burns of the hand. Plast Reconstr Surg. 1990;86(5):935-40. Available from: https://doi.org/10.1097/00006534-199011000-00016
- Yamashita S, Sato S, Kakiuchi Y, Miyabe M, Yamaguchi H. Lidocaine toxicity during frequent viscous lidocaine use for painful tongue ulcer. J Pain Symptom Manage. 2002;24(5):543-5. Available from: https://doi.org/10.1016/s0885-3924(02)00498-0
- Public Health Advisory: Life-Threatening Side Effects with the Use of Skin Products Containing Numbing Ingredients for Cosmetic Procedures. 1/2009: For current information on this issue. 2/6/2007. Available from: https://ohsonline.com/articles/2009/01/19/19-fda-issues-public-health-advisory-alert-on-skin-numbing-products.aspx?m=1
- Elad S, Raber-Durlacher JE, Brennan MT, Saunders DP, Mank AP, Zadik Y, et al. Basic oral care for hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT). Support Care Cancer. 2015;23(1):223-36. Available from: https://doi.org/10.1007/s00520-014-2378-x